- Info
Forschung
Molekulare Medizin - AG Reinheckel
We are interested in a quite fundamental decision, made by any cell being a part of a multi-cellular eukaryotic system: whether it will continue to live, or die—for the benefit of the organism.
This decision is genetically determined: the cell death program of apoptosis is, just as cell growth, proliferation and differentiation, an integral component of life. Apoptosis is absolutely required to facilitate and organize e.g. development, immune responses or organ renewal. In addition to apoptosis, other forms of programmed cell death, such as necroptosis, have been discovered, while their physiological role is not well understood.
Cell death signaling pathways are controlled by reversible posttranslational modifications of signaling proteins, such as phosphorylation, acetylation and ubiquitylation, and lastly by proteolysis, representing an irreversible posttranslational modification. My group aims at understanding the role of these posttranslational modifications in detail. We are working on two main projects, focusing on the intrinsic apoptosis pathway, and secondly on TNF-induced inflammation, apoptosis and necroptosis. |
1.) Regulation of the expression and activity of BCL-2 family
members by GSK-3
|
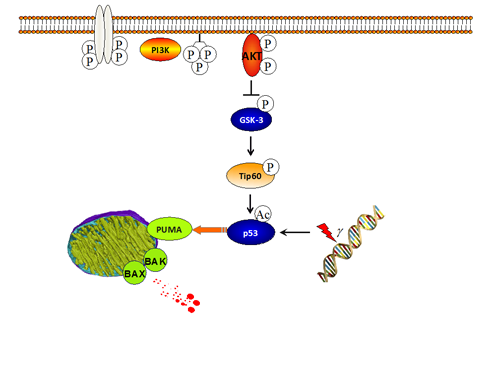 |
Stimulation of growth factor receptors mediates cell growth and proliferation, but it also generates pro-survival signals in the cell, through, for example, MAPKinase or PI3Kinase pathways—the machinery controlling programmed cell death, apoptosis, is under the control of growth factor signaling.
PI3K signaling activates the anti-apoptotic kinase AKT, which in turn inactivates the two isoforms of GSK-3, the kinases GSK-3 and GSK-3, by inhibitory phosphorylation. We are investigating the death-promoting activities of GSK-3. We previously showed that the antiapoptotic BCL-2 family member MCL-1 is subject to phosphorylation by GSK-3, and we later showed that this affects leukocyte numbers in mice.
More recently, we demonstrated that GSK-3 is required for the induction of cell death by the tumor suppressor p53, which is mediated by the pro-apoptotic BCL-2 protein PUMA. We could show that GSK-3 phosphorylates and activates of the acetyltransferase Tip60, resulting in p53 acetylation and PUMA induction upon DNA damage.
Phosphorylation of Tip60 by GSK-3 requires a priming phosphorylation, and we are currently exploring the candidate kinases mediating this phosphorylation, as well the effects of this phosphorylation on the affinity of Tip60 to chromatin, pointing to a more general role of this phosphorylation in the regulation of transcription. In addition, we are currently investigating the interplay of Foxo3a and GSK-3 in the control of PUMA and apoptosis, induced by growth factor withdrawal. |
| Relevant recent publications: |
Lindner, S. E., Wissler, M., Gründer, A., Aumann, K., Ottina, E., Peintner, L., Brauns-Schubert, P., Preiss, F., Herzog, S., Borner, C., Charvet, C., Villunger, A., Pahl, H. L., and Maurer, U. (2014) Increased leukocyte survival and accelerated onset of lymphoma in the absence of MCL-1 S159-phosphorylation. Oncogene 33, 5221–5224 |
Charvet, C., Wissler, M., Brauns-Schubert, P., Wang, S.-J., Tang, Y., Sigloch, F. C., Mellert, H., Brandenburg, M., Lindner, S. E., Breit, B., Green, D. R., McMahon, S. B., Borner, C., Gu, W., and Maurer, U. (2011) Phosphorylation of Tip60 by GSK-3 Determines the Induction of PUMA and Apoptosis by p53. Mol Cell 42, 584–596 paper highlighted in: Mol Cell (2011) 42, 555-556
|
2.) Regulation of TNF-induced cell death and inflammation
|
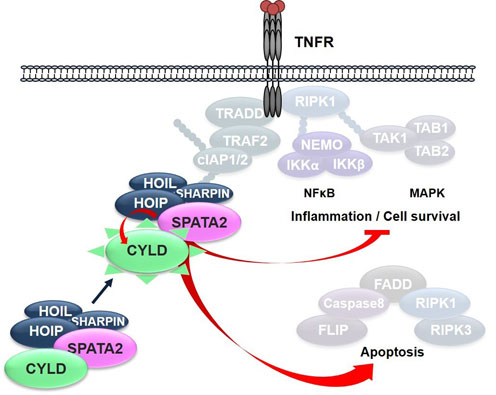 |
Apoptosis, necropotosis and the expression of inflammatory cytokines, induced by the cytokine TNF, are regulated by reversible nondegradadative ubiquitylation. Most prominently, K63-linked ubiquitylation, conferred by E3 ligases such as the IAPs, and linear (M1-linked) ubiquitylation, mediated by the LUBAC complex, influence the decision between cell death and survival upon TNF receptor stimulation. Ubiquitylation is counteracted by deubiquitinases, which remove ubiquitin chains by proteolysis. In this project, we are investigating the regulation of the deubiquitinase CYLD by phosphorylation, and the interaction of CYLD with other proteins.
We have identified the protein SPATA2 as protein interacting with CYLD, as well as the LUBAC complex, which mediates linear ubiquitylation. We thereby defined SPATA2 is a novel constituent of the TNF receptor signaling complex, promoting TNF-induced cell death. We are currently further exploring the role of SPATA2 for programmed cell death, and inflammatory responses in vivo. |
| Relevant recent publication: |
Schlicher, L., Wissler M., Preiss, F. Brauns-Schubert, P., Jakob, C., Dumit, V., Borner, C., Dengjel, J., Maurer, U. (2016): SPATA2 promotes CYLD activity and regulates TNF-induced NF-κB signaling and cell death EMBO Rep in press |
|
-
Juli
| Mo | Di | Mi | Do | Fr | Sa | So |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | 31 | | | | |
|