- Info
Forschung
Unbenanntes Dokument
| |
|
|
| |
Research interests (nur englisch) |
|
| |
Our laboratory focuses on intracellular signalling pathways and how their intricate regulation is disturbed in human diseases and is influenced by clinically relevant drugs. Currently, we are working on three project areas:
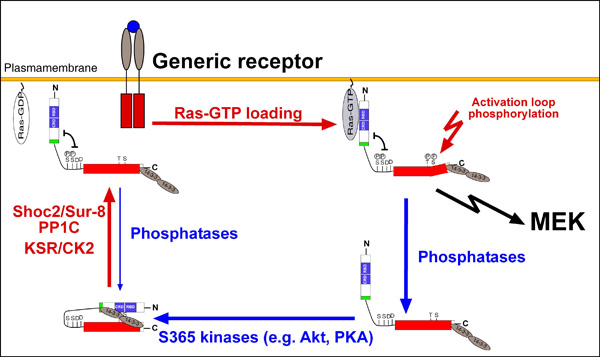 Fig.1. click to enlarge
Simplified sketch of the B-Raf activation cycle
Fig.1. click to enlarge
Simplified sketch of the B-Raf activation cycle (taken from Brummer, 2008)
|
|
| |
| 1. |
A comprehensive and functional analysis of B-Raf signalling (funded by the Emmy-Noether-Program of the DFG) The serine/threonine kinase B-Raf represents an important oncoprotein, which is mutated in about 8 % of all human cancer, with mutation rates rising up to 90 % in certain tumour entities (Röring & Brummer, 2012). Even in the absence of mutations, B-Raf is often dysregulated due to the mutation and/or over-expression of its activators such as Ras or receptor tyrosine kinases (RTKs). B-Raf is regulated by multiple phosphorylation events provided by upstream activators and by crosstalk with other pathways and feedback loops. Using phospho-proteomics, we are currently working towards a more dynamic and refined model of the B-Raf activation cycle (Fig. 1). B-Raf activity is strongly regulated by homo-dimerisation or by heterodimerisation with other Raf-isoforms or KSR proteins. Recently, we have characterised the importance of dimerisation for wildtype, oncogenic and drug-inhibited B-Raf. For example, we could show that the formation of B-Raf homo- and heterodimers is based on distinct structural requirements and that oncogenic B-Raf mutants differ from wildtype B-Raf by forming particularly stable homo-dimers. We also demonstrate that an intact dimer interface is pivotal for the paradoxical action of Raf inhibitors, a phenomenon linked to side-effects and drug resistance phenomena (Röring et al., 2012). We are also interested in the molecular mechanisms by which novel tumour-associated BRAF mutations cut the aforementioned activation cycle short and contribute to tumourigenesis (Eisenhardt et al. 2011; Cin et al., 2011). |
|
| 2. |
Mechanisms behind the oncogenic activity of B-Raf mutants (supported by the Collaborative Research Centre (CRC) 850, Project B4) Another interest of the laboratory are the downstream signalling events by which oncogenic B-Raf drives malignant transformation. So far, this oncoprotein has been mainly implicated as a driver of proliferation, survival and angiogenesis. In this CRC project, we characterise its contribution to other hallmarks of cancer and prerequisites for metastasis, such as increased motility and the epithelial-to-mesenchymal transition (EMT). To this end, we have generated several isogenic human epithelial cell line models, which allow us to express the oncoprotein conditionally. For example, we have introduced a novel doxycycline (dox)-regulated expression system into MCF-10A mammary epithelial (Fig. 2) and Caco-2 colon adenocarcinoma cells. Using these systems, we are currently studying the impact of oncogenic B-Raf on various aspects of epithelial cell biology in conventional tissue culture and 3D culture systems (Herr et al., 2011; Fritsche-Günther et al. 2011; Röring et al. 2012).
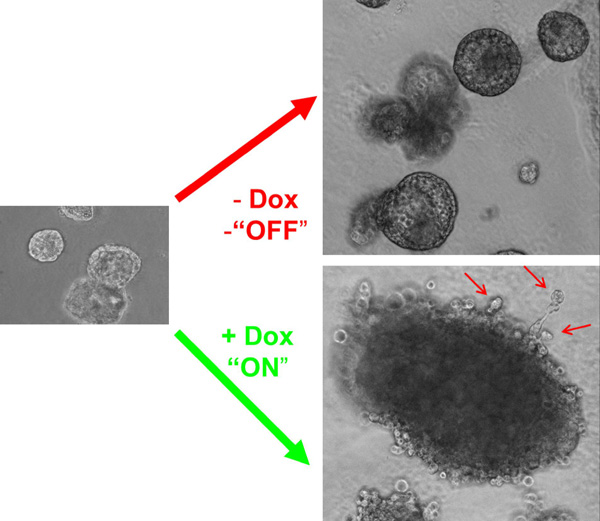 click to enlarge
Fig.2 Doxycylin (dox)-induced expression of oncogenic B-Raf in immature acini (left) prevents normal development of MCF-10A cells into acinar structures in 3D culture.
click to enlarge
Fig.2 Doxycylin (dox)-induced expression of oncogenic B-Raf in immature acini (left) prevents normal development of MCF-10A cells into acinar structures in 3D culture. (Taken from Herr et al. 2011) |
|
| 3. |
The role of the Gab2 docking protein in tumour development and drug resistance (supported by the DFG-funded Spemann Graduate School for Biology and Medicine and the Research Seed Capital program of the Ministry for Arts and Sciences, Baden-Württemberg) Docking proteins of the Grb2-associated binder (Gab) family represent hubs in tyrosine kinase signalling networks. Following their recruitment to the plasma-membrane, these proteins become tyrosine phosphorylated and then recruit effector molecules with SH2 domains such as the tyrosine phosphatase SHP2 or the regulatory subunit of PI-3K, p85. This leads to the modulation and amplification of several downstream pathways involved in proliferation and survival (Wöhrle et al. 2009). Gab proteins are subject to a complex pattern of positive and negative feedback regulation that modulates their signalling output and oncogenic potential (Brummer et al. 2008). There are three Gab proteins in mammals, Gab1-3. We are particularly interested in the Gab2 isoform, which is increasingly implicated in several human malignancies such as breast and ovarian cancer, melanoma and chronic myelogenous leukemia (CML). In that regard, we have recently shown that the number of Gab2 positive cells increases during CML progression (Aumann et al. 2011) and that Gab2 signalling confers drug resistance to CML cells to clinically relevant Bcr-Abl inhibitors such as imatinib, nilotinib and dasatinib (Wöhrle e al. 2012).
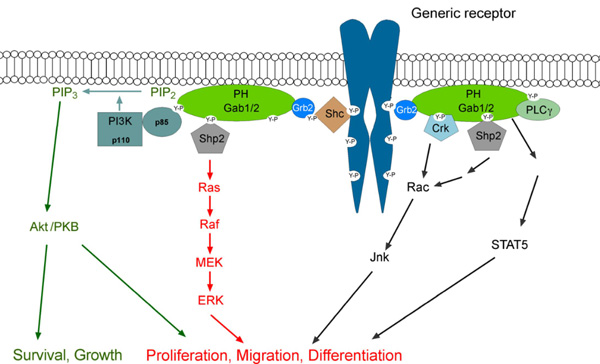 click to enlarge
click to enlarge
Fig.3 The Gab2 signalling network. (Taken from Wöhrle et al. 2009) |
|
| |
|
|
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
|
-
Juli
| Mo | Di | Mi | Do | Fr | Sa | So |
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| 29 | 30 | 31 | | | | |
|




